In a paper posted last thirty day period in Nano Letters, the group explain how they’ve created a novel “fireproof” strong-state electrolyte (SSE) for use in lithium-ion batteries. “We address the trouble of flammability in SSEs by incorporating a fire retardant,” suggests Jiayu Wan, a postdoctoral researcher in Cui’s lab and co-author of the paper.
They made use of a flame-retardant product referred to as decabromodiphenyl ethane, or DBDPE for shorter. To make their new strong-state electrolyte, the group 1st created a skinny film by combining DBDPE with polyimide, a mechanical enforcer.
Using polyimide has quite a few advantages, suggests Wan. Apart from being “mechanically truly robust,” it features a superior melting point (making it significantly less very likely that a shorter circuit will come about), a remedies-based manufacturing system (which is compatible with how batteries are manufactured currently), and it’s cheap (3M even has film tapes manufactured from it).
The hitch, however, is that polyimide simply cannot conduct ions. To get close to this snag, Wan and his colleagues additional two unique polymers, polyethylene oxide (PEO) and lithium bistrifluoromethanesulfonylimide (LiTFSI), to the blend.
“It’s innovative—they’ve well made use of co-polymers, which is a new way to clear up the flammable polymer electrolyte battery trouble,” suggests Chunsheng Wang, a researcher who scientific tests new battery technologies at the College of Maryland.
Reliable-state electrolytes choose two key types. You can make them from ceramics, a product that conducts ions properly but is extremely brittle and outcomes in thick batteries, which have lessen strength density. Or, you can have electrolytes composed of polymers, which are small charge, light-weight, and flexible. They’re also “soft,” meaning there’s small resistance alongside the interface of the electrode and electrolyte, which enables the electrolyte to conduct ions easily.
But polymer electrolytes also have problems. “This softness indicates they’re unable to suppress lithium dendrite propagation, so they’re flammable,” suggests Wang, referring to the small needle-like projections that increase from a battery’s anode. Dendrites can result after recurring cycles of charging and discharging when these lithium crystals pierce a battery’s separator, they can start off fires.
“A lot of people today believe that for liquid electrolytes, there is no resistance and dendrites can increase by way of the electrolyte,” suggests Wang. “But if you change the liquid with a strong, which is mechanically much better, the lithium may be blocked.”
Their mechanical strength, alongside with reduced flammability, are just some motives why strong-state electrolytes have garnered interest between researchers in both academia and sector. A 3rd reason lies with the fact that they make it possible for batteries to be stacked. “Because the electrolyte doesn’t movement, you can easily put them jointly without the need of wires… which is significant for rising strength density,” suggests Wang.
There’s no excellent solution, nevertheless. “All the unique SSEs have some problems, so you have to stability them out,” he suggests.
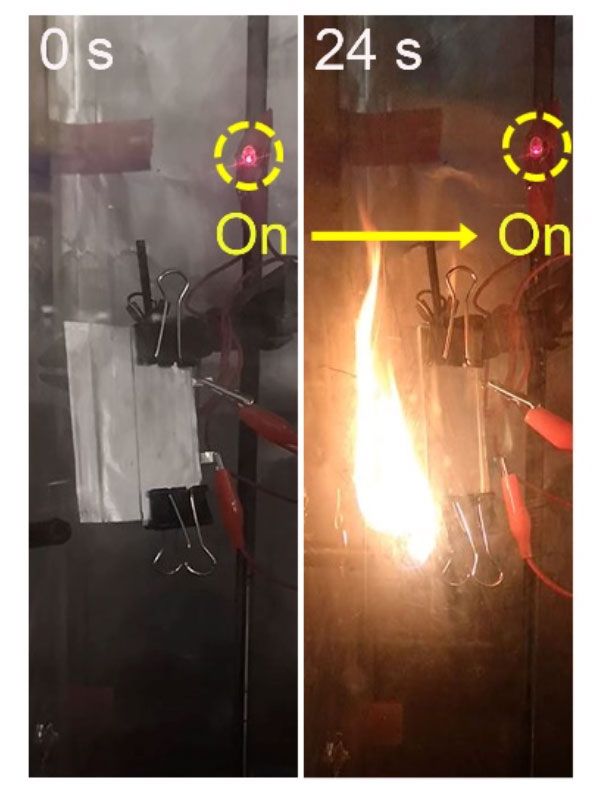
It is a purpose that the group at Stanford appears just one stage nearer to achieving. Not only is their new strong-state electrolyte ultrathin (measuring concerning 10 to twenty five micrometers), it also provides a superior certain potential (131 milliampere hours for every gram, mAh/g, at one degree C), and demonstrates good biking overall performance (long lasting 300 cycles at sixty levels C). Crucially, prototype battery cells manufactured employing it proved to operate in spite of catching fire (in this movie, an LED continues to be lit even nevertheless the battery powering it is on fire).
“This was quite shocking to us,” suggests Stanford’s Wan. “Usually a battery will just explode with a fire. But with this just one, not only does it not explode, it however functions.”
Nowadays, the group proceeds to explore new materials and structures for use in strong-state electrolytes, with the intention of enhancing latest density and mobile potential. Suggests Wan: “The obstacle now is to make the battery charge a lot quicker, have a better strength density, and to last for a longer time.”





More Stories
MP3 Players – Knowing the Various Interesting Facts About These Gadgets
How Hotels Can Cope Up With the Airbnb Generation Through Information Outsourcing
Roaring Machines – James Bond Vehicles